AMAI (formerly Medichain) bring you the CLDC Diagnostic test, a cost-effective and reliable algorithm based diagnostic tool aimed at detecting a wide range of diseases and illnesses. The test utilizes blood markers to predict and help manage conditions such as Ischemic heart disease, Stroke, and Chronic obstructive pulmonary disease (COPD), as well as problems like leukemia, pneumonia, HIV infection, sepsis, and tuberculosis, among others. It can also identify viral infections such as COVID-19, mononucleosis, and mumps, and detect chemotherapy side effects, radiation exposure, and skin infections. The test’s eosinophil and basophil markers can detect allergic reactions, parasitic infections, and acute allergic reactions. Additionally, the test may have the potential to detect the presence of Nipah virus, SARS, and MERS, which are considered possible leaders for future global pandemics. With this test, patients can conveniently and quickly test themselves at home, enabling early diagnosis and timely treatment, ultimately improving patient outcomes.
The founder of AMAI (Advanced Medical Artificial Intelligence) formed a company and led the team that won the Global Coronahack against 1000 laboratories and institutions worldwide using this method. Subsequently, they received UK government backing to develop a blood testing method that allows for a level of diagnosis surpassing that of PCR, while maintaining lower costs per test compared to lateral flow and antigen tests. Research findings demonstrate that when correctly applied, this form of testing can effectively halt the spread of COVID-19 in days, without the need for lockdowns, mass isolation, or quarantine, significantly reducing the social, economic, and human costs of COVID. Furthermore, the same method shows potential in combating nearly thirty of the most significant global killers.
We can now offer large organizations, health systems, and governments the means to conduct rapid mass testing, which can have a significant impact on diseases and conditions that claim the lives of tens of millions of people every year. For COVID, the first use case, the efficiency of this test has been demonstrated, surpassing the performance of any other test by a factor of 2000% or more, as indicated by major EU trial data. If there were to be a new vaccine-resistant strain and this system was implemented, we could effectively stop the spread of COVID.
3 minute pinprick blood test includes results for the following
But now it goes beyond COVID. There are dozens of major killers we can impact.
| COVID-19 by CLDC | Hepatitis B | Leukaemia Nonalcoholic Fatty Liver Disease | Anemia |
|---|---|---|---|
| Early inflammatory response neutrophils markers for | Lymphocytes markers for | Monocytes markers for | Eosinophils markers for |
| Acute stress | Chronic infection | Chronic inflammatory disease | An allergic reaction |
| Gout | Mononucleosis | Tuberculosis | Parasitic infection |
| Rheumatoid arthritis | Leukaemia | Viral infection, such as measles, mononucleosis, and mumps | Basophils markers for acute allergic reaction |
| Thyroiditis | Viral infection, e.g. mumps or measles | Bloodstream infection | |
| Trauma | Chemotherapy side effects | Chemotherapy side effects | |
| Pregnancy | HIV infection | Bone marrow disorder | |
| Anemia | Sepsis | Skin infections | |
| Bacterial infection | Radiation exposure, either accidental or from radiation therapy | ||
| Chemotherapy side effects | |||
| Influenza or other viral illnesses | |||
| Radiation exposure |
AMAI’s CLDC system handles COVID variants better than other tests.
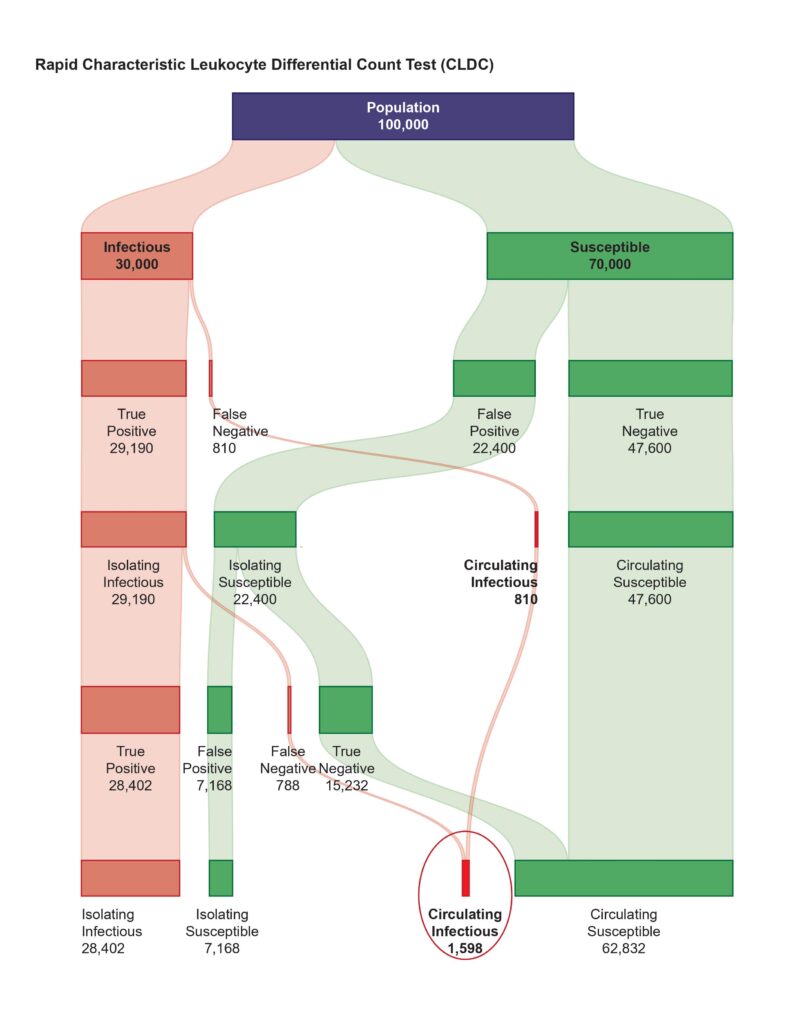
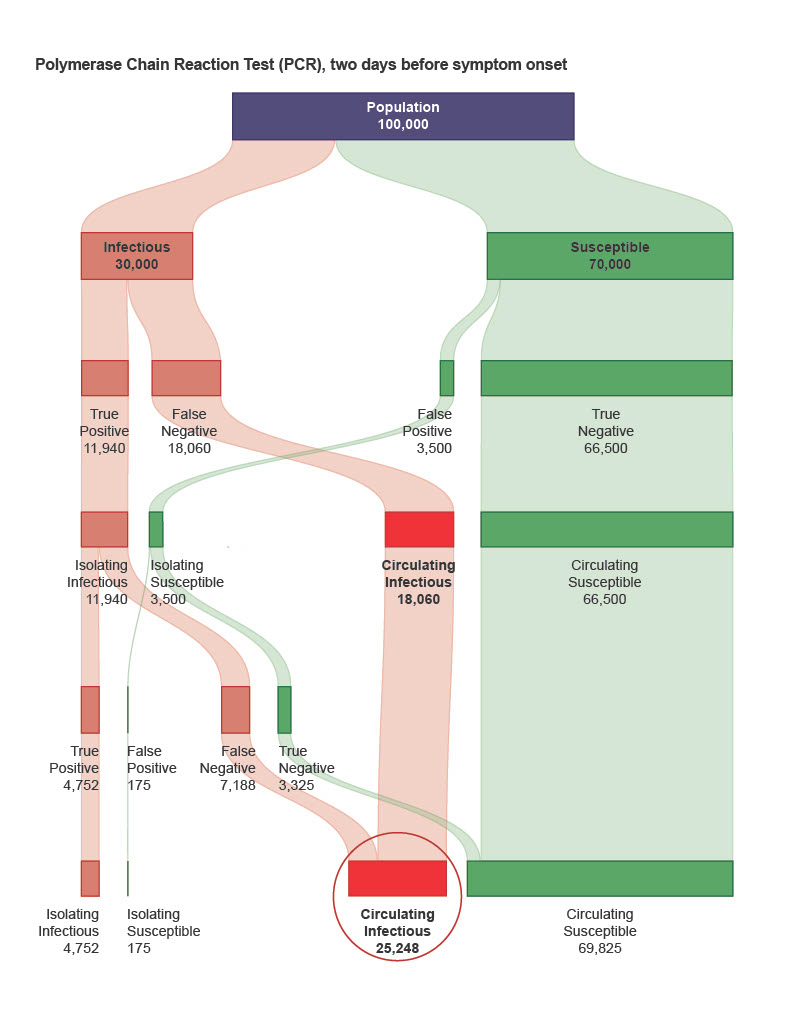
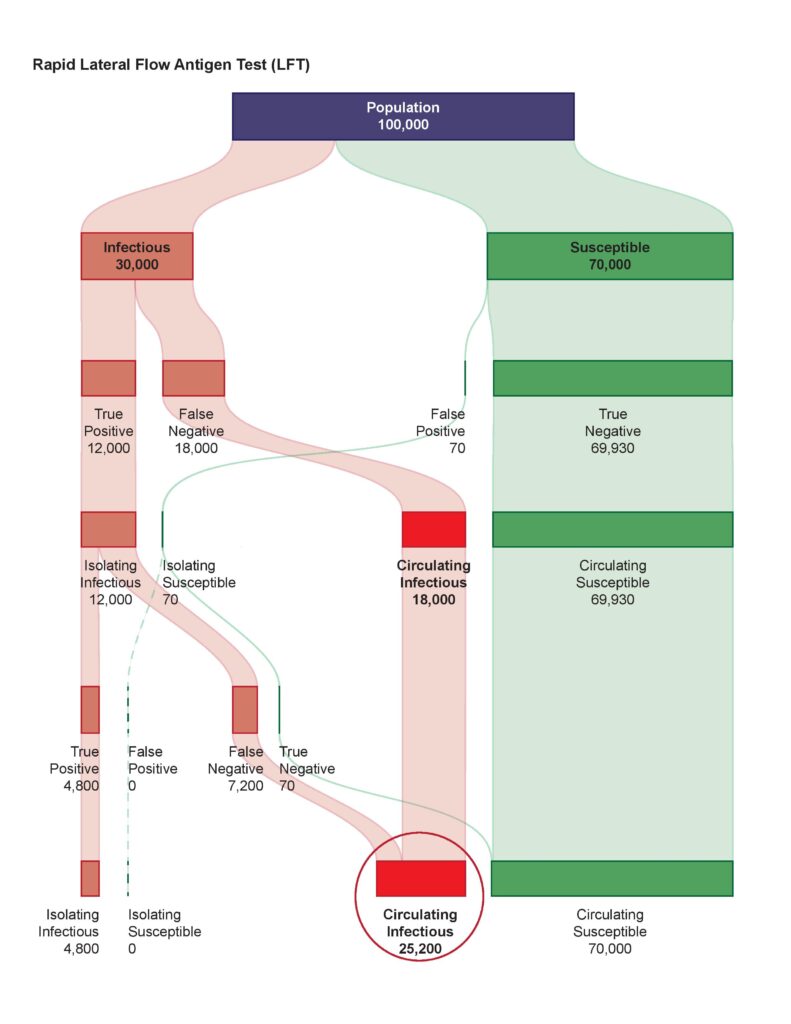
Designed by a former Oxford University cancer scientist, the home version shown by the BBC involves no labs or reagents, while the desktop version can be used in schools, hospitals, factories, border control and carried out a blood test indictive of dozens of crucial everyday conditions as well as COVID. A top pharmaceutical company chief is quoted as saying that it could easily offer sixty millions of tests per day in an EU country or UK.
Crucially it has the potential to diagnose COVID-19 up to two weeks earlier than other tests, with 98.7% sensitivity and high specificity, allowing individuals to isolate before infecting others and removing the need for community-wide restrictions and lockdowns.